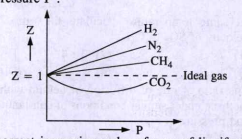
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
4.6 (148) · $ 15.99 · In stock

y factor Compressibility factor 2 V is plotted agalnst pressure RT What is the correct order of correct order of liquet ability of the gases shown in the above graphi (5) Hz

Compressibility factor - Wikipedia

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H
When does real gas behave as ideal gas? - Quora

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor - Wikipedia

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

Explain the shape of graph obtained between pressure P and 1/v for perfect gas at constant temperature? - Quora
1.5 Real Gases and the Virial Equation - Mail

EngArc - L - Compressibility Factor

Compressibility factor Z - Gaseous State

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C
Consider a graph between compressibility factor Z and pressure P











