- Home
- compressibility factor equation
- If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
4.7 (306) · $ 22.50 · In stock

If Z is a compressibility factor, van der Waals equation at low pressure ..

Jee main-2014-solution-code-h-english

Jee main-2014-solution-code-h-english
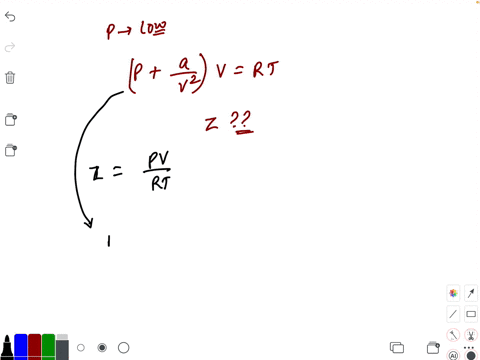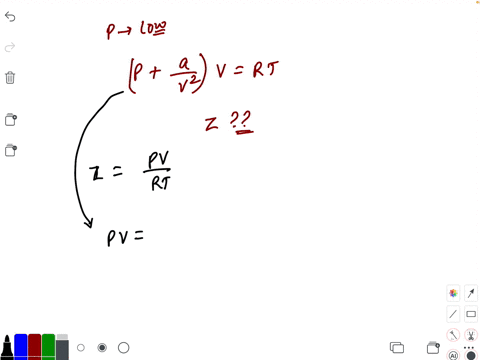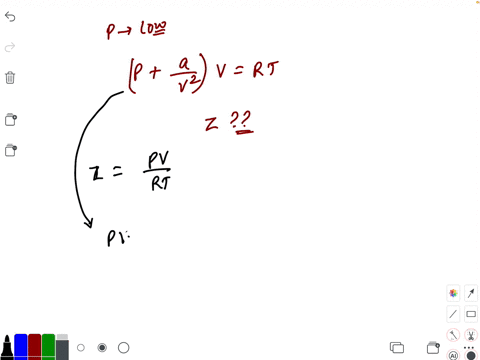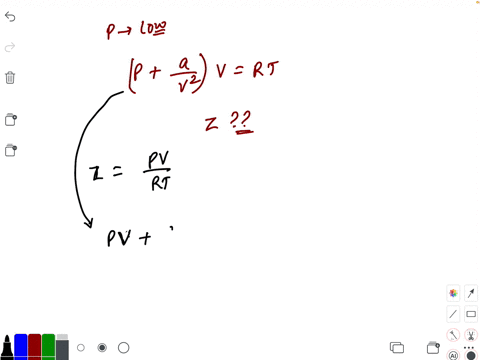
⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

If Z is a compressibility factor, van der Waals' equation at low press

66. If z is the compressibility factor, van der Waals equation low pressure can be written as: (A) Z = 1 + PT (B) 2 = 1 - VT (C) 2=1 - (0) 2 =1+ PT Space rough use

JEE Main 2014 (Offline) JEE Main Year Wise Previous Years Questions - ExamSIDE.Com

Gaseous State Questions for JEE exam - Free Online All questions of Gaseous State - Chapter-wise Questions of JEE
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0°C - Sarthaks eConnect












