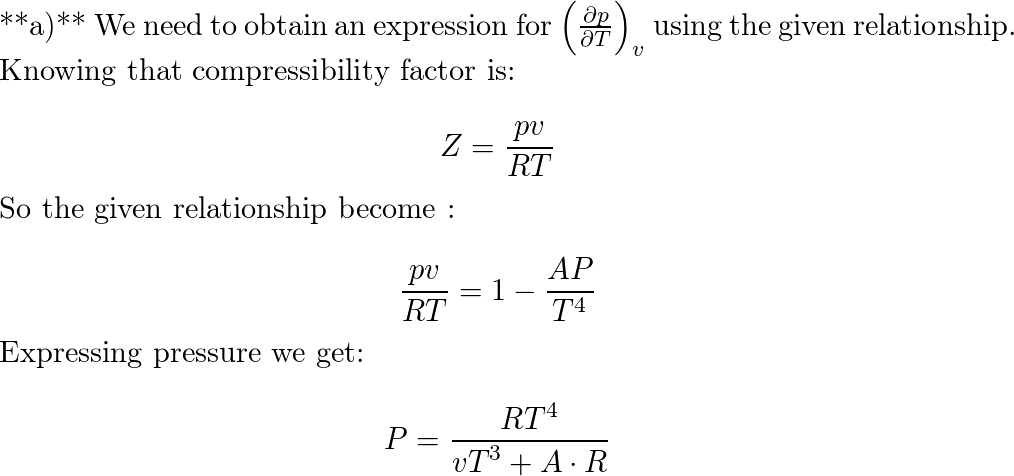- Home
- compressibility factor equation
- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
4.7 (358) · $ 25.50 · In stock
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

The compressibility factor of a van der Waals gas the critical point is equal to

Solved 9 Compression factor Z Use the van-der-Waals equation

compressible flow related terms - Department of Mechanical and

The compressibility factor for a real gas at high pressure is
for a real gas at 25∘C temperature and high pressure (99 bar) the value o..

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

Solved Task 3 For the van der Waals gas, the compression

If Z is a compressibility factor, van der Waals equation at low pressure ..

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

At a high pressure, the compressibility factor (Z) of a real gas is us
PDF) Measurement and Prediction Method of Compressibility Factor at High Temperature and High Pressure

SOLVED: The van der Waals and Redlich-Kwong Equations, which are used in high-pressure conditions, are as follows: The van der Waals equation: (P + a(n/V)^2)(V - nb) = nRT where: P