physical chemistry - Is the compressibility factor smaller or
4.8 (375) · $ 15.99 · In stock
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Real Gas Behavior The Compression Factor (Z) [Example #2]

Real Gases Introductory Chemistry

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Compressibility Factor of Gas Overview, Equation & Chart

3.2 Real gas and compressibility factor – Introduction to
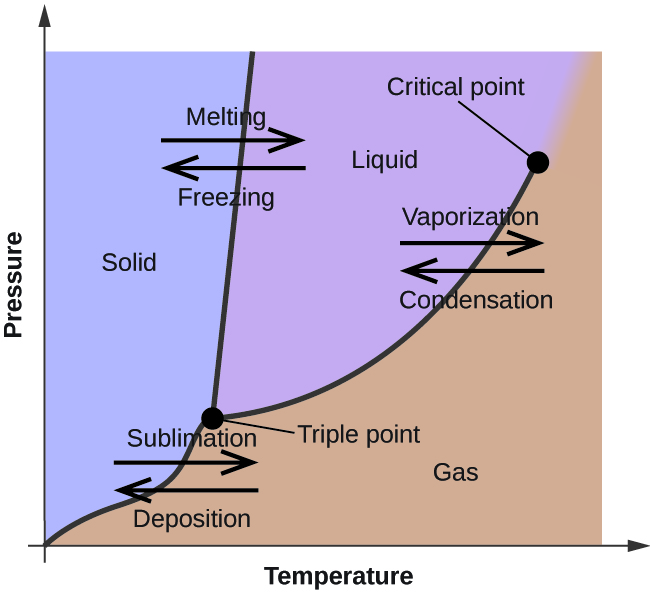
Phase Diagrams Chemistry for Majors
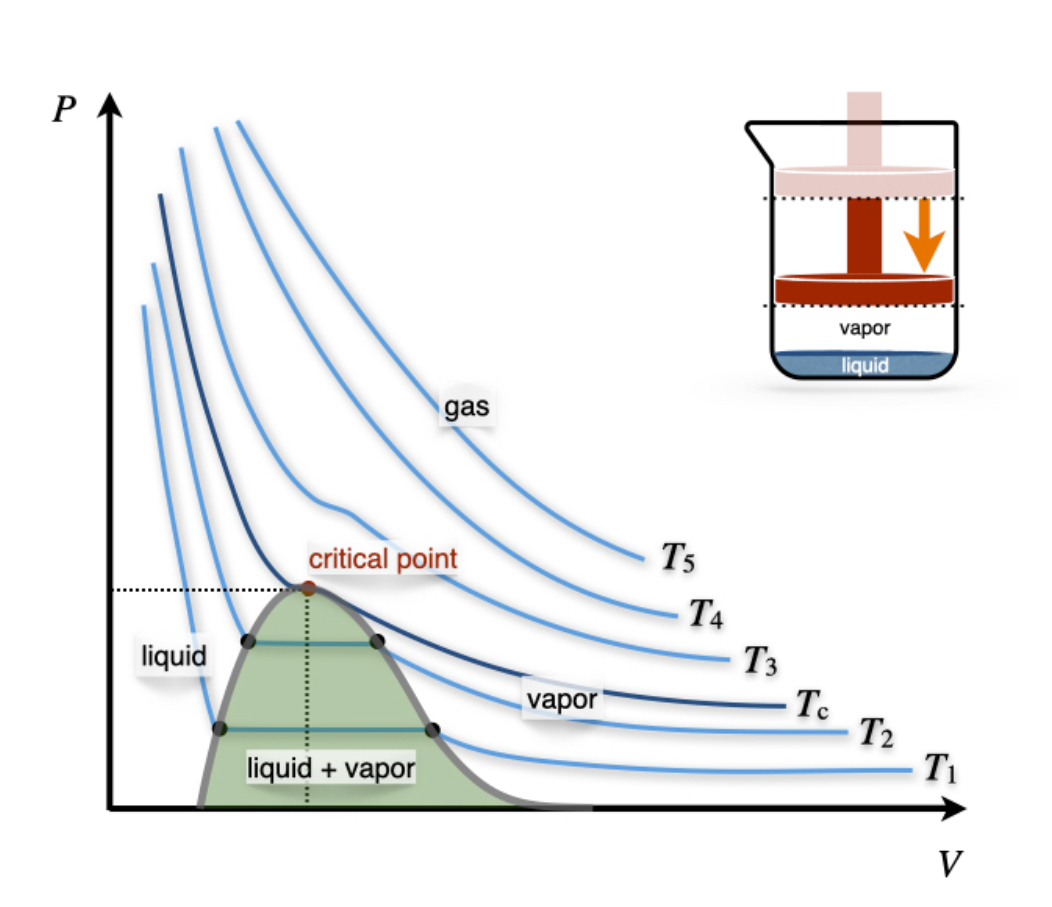
11.3: Critical Phenomena - Chemistry LibreTexts

Sound, Properties, Types, & Facts

Class Notes on Compressibility of a Real Gas, CH 417

Cubic equations of state - Wikipedia

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
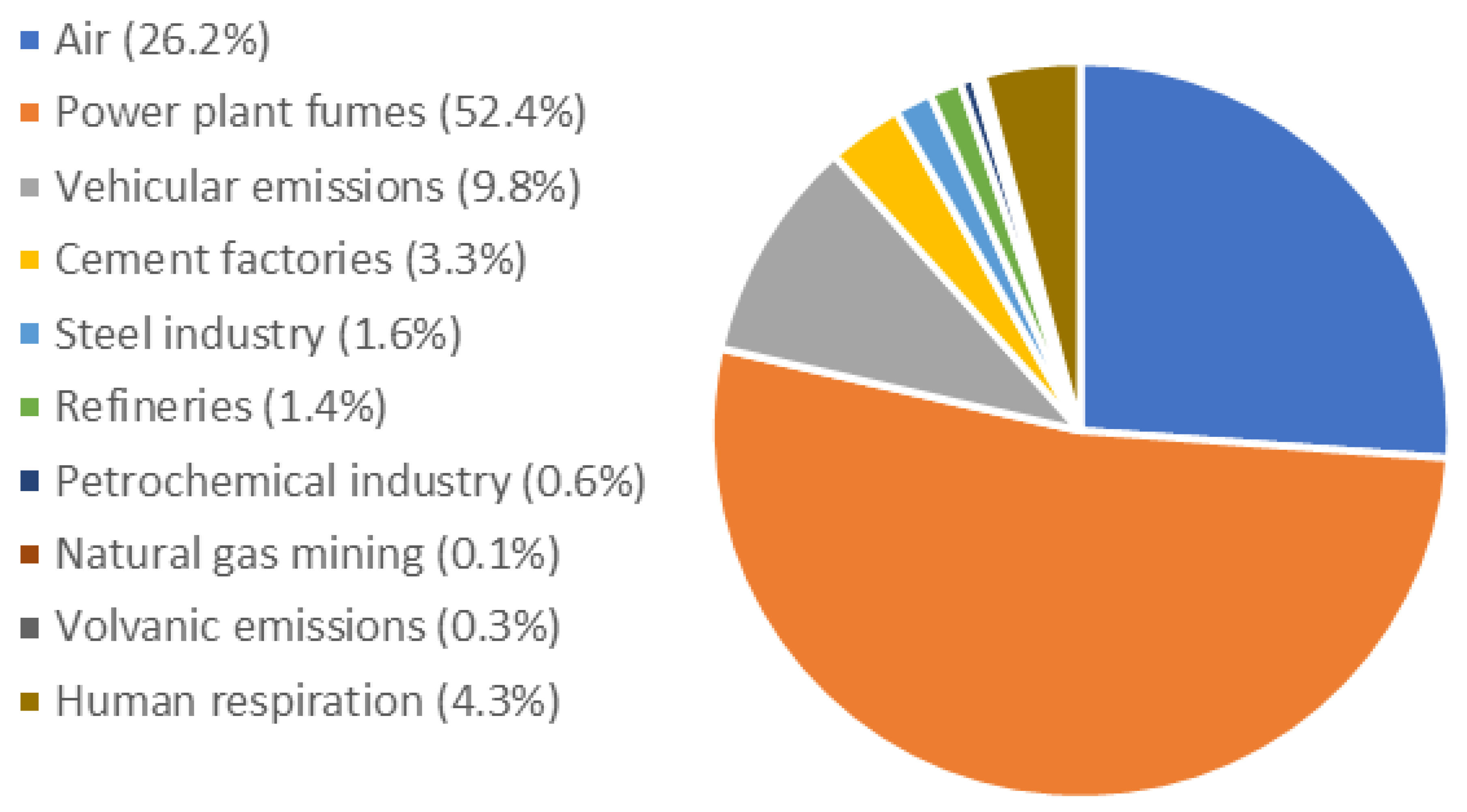
Atmosphere, Free Full-Text












