Medtronic Insulin Pump Devices Recalled Due to Serious Risks
4.7 (476) · $ 7.99 · In stock
The Food and Drug Administration on Tuesday issued a warning notifying patients that medical device maker Medtronic has expanded a recall of remote controllers for

Medtronic MiniMed Insulin Pump Lawsuit, Whitley Law Firm

Medtronic Minimed Insulin Pump Recall Issued Over Cybersecurity

Cynerio Launches IoT Device Security Solution for Small Hospitals

Medtronic MiniMed Insulin Pump Recall Leads to Lawsuits Filed

Medtronic Recalls MiniMed Insulin Pumps for Incorrect Dosing Risk
Medtronic Nabs a Sweet FDA Approval in Diabetes
Medtronic's new insulin pump gains FDA approval
Medtronic Recalls Mini Med and Pro Infusion Insulin Pumps due to

Medtronic facing class action suit from Canada MiniMed insulin

FDA: Certain Medtronic insulin pumps recalled due to cybersecurity

Medtronic Insulin Pump Devices Recalled Due to Serious Risks
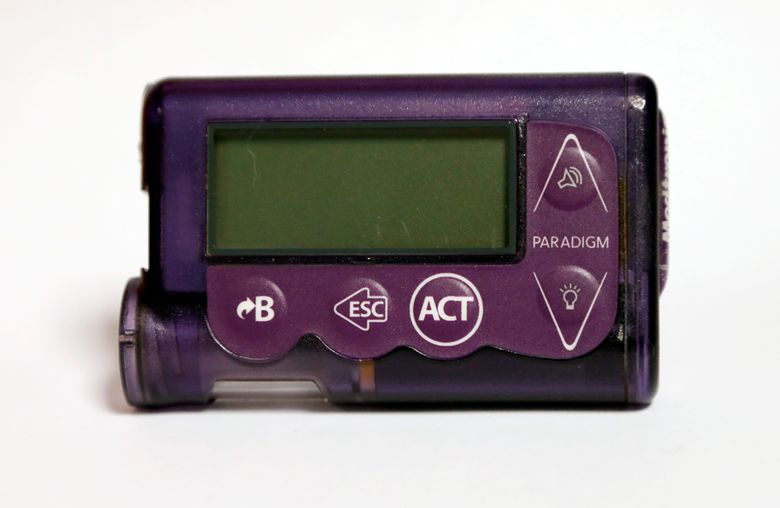
Insulin pumps, widely used, also have the most problems in FDA's

Medtronic Recalls Insulin Pumps Over Concerns For Hacking Risk
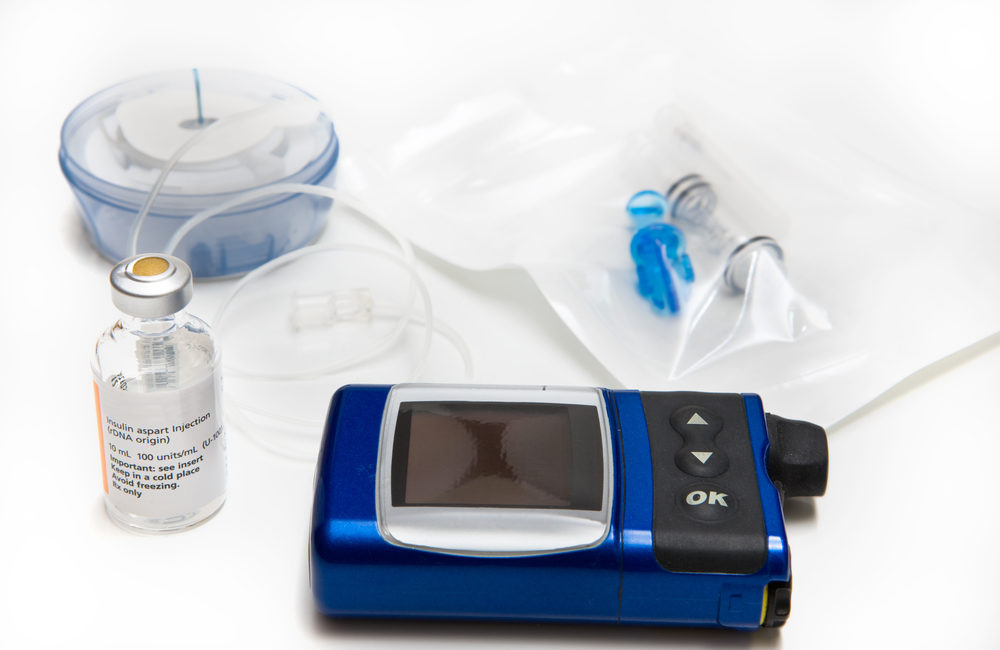
FDA Recalls Medtronic Insulin Pumps Due to Hacker Risk - Drug And

Safety alerts Medtronic Diabetes